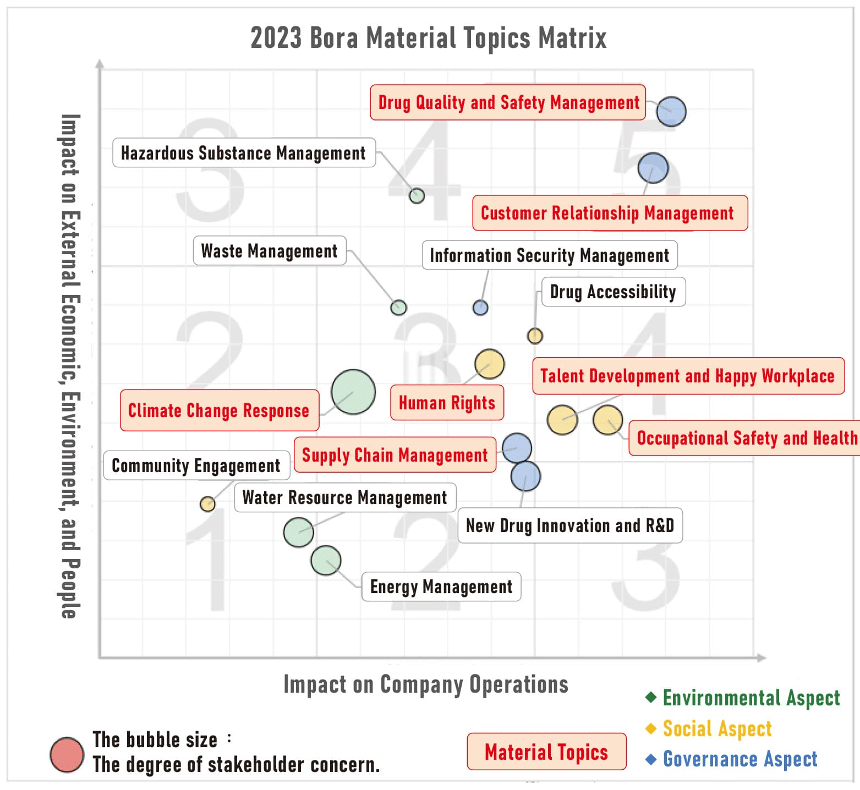
| |
Material Topics |
Description of Material Topics |
Management Guidelines |
| Environment |
Climate Change Response |
Explanation of potential events, their risks, likelihood of occurrence, and response plans |
Refer to 4.1 Climate Change Response |
| Social |
Talent Development and Happy Workplace |
Establish competitive and fair compensation and benefits policies, providing various allowances and benefits |
- The company provides cross-departmental, cross-company, and even international rotation opportunities, along with various development plans, allowing employees to develop according to their aptitudes
- Strive to enhance open and transparent communication channels between supervisors and employees, and among employees, to promote labor-management harmony and create a win-win situation for the company and employees
- Promote various activities and clubs, providing related subsidies to help employees achieve work-life balance
|
| Occupational Safety and Health |
The company has established relevant management measures to ensure the occupational safety and health of employees and suppliers |
- To ensure the safety and health of employees in the workplace and the occupational safety and health of employees, the company has established the responsibilities of supervisors, commanders, supervisors, and all employees (including contractors) in implementing labor safety and health management according to law. Plant facilities’ public health and cleaning procedures are established to maintain good public health and reduce the possibility of product contamination, complying with environmental regulations
- The company implements relevant measures according to occupational safety and health laws, establishes automatic inspection plans, occupational safety and health management plans, and related management methods to ensure employee protection during operations
- The company regularly conducts internal audits to confirm the safety and health implementation status of each plant and area, ensuring compliance with legal requirements
|
| Human Rights Protection |
Formulate relevant human rights protection and labor policies and implementation measures |
- Abide by international human rights conventions such as the “Universal Declaration of Human Rights,” “UN Global Compact,” and “International Labour Organization Conventions,” and eliminate any infringement and violation of human rights
- Ensure that all employees of the company receive fair, equal, and dignified treatment
- This policy applies to Bora Pharmaceuticals Co., Ltd. and all its affiliated companies
|
| Governance |
Supply Chain Management |
Follow internal procedures for supplier management, ensuring the safety of company employees and contractors is well-protected; creating a win-win-win situation for Bora, suppliers, and customers |
- For critical raw materials or services, establish more than two suppliers with mutual substitutability and competitiveness to diversify supply risks and reduce costs
- Supplier selection criteria include quality system certifications, on-site audit results, or official/third-party audit history, etc.
- Conduct annual quality reviews for suppliers and regularly re-evaluate quality documents or conduct on-site audits for qualified suppliers
|
| Drug Quality and Safety |
Follow government pharmaceutical regulations and meet PIC/S GMP certification standards to ensure that customer medications comply with all standards, ensuring drug safety and manufacturing compliant products |
- Regularly assess regulatory trends and take corresponding measures in advance
- Meet official inspection standards of the Taiwan Ministry of Health and Welfare, the U.S. Food and Drug Administration (FDA), and others
- Conduct irregular inspections to meet customer requirements and ensure compliance
- Regularly participate in seminars and courses held by the Ministry of Health and Welfare’s Food and Drug Administration, review the plant’s quality systems and related standard operating procedures according to regulatory updates, and adjust operations to comply with regulations
- To achieve this quality goal, the company has a comprehensively designed and correctly implemented pharmaceutical quality system. This system covers Good Manufacturing Practices (GMP) and quality risk management, which should be fully documented and monitored for effectiveness. All departments in the pharmaceutical quality system should be appropriately staffed with competent personnel and equipped with adequate facilities, equipment, and infrastructure. Manufacturing license holders and authorized persons have additional legal responsibilities
|
| Customer Relationship Management |
The quality of the company’s customer service, results of customer satisfaction surveys, and improvement measures. Implement customer service processes, enhance staff training, and improve customer satisfaction. |
- Conduct product education training for sales staff to enhance overall professionalism
- Regional supervisors conduct joint visits to accurately understand customer needs
- Continuously refine to the highest standards to protect customer data and their rights
|