Management Approach
Bora Pharmaceuticals is committed to building a stable, sustainable supply chain to support long-term goals. By sourcing alternative materials, diversifying suppliers, and strengthening management, we enhance supply chain resilience. Supplier selection focuses on quality certifications (e.g., GMP, ISO 9001), on-site audit results, and third-party audit records. Additionally, we ensure key raw materials are interchangeable and competitive to reduce supply chain risks and improve efficiency.
Supplier Management Approach
Bora ensures supply stability and sustainability through a diversification strategy- engaging, at least 2 interchangeable, competitive suppliers for critical materials and services to mitigate risks and control costs.
Supplier Selection Criteria Include:
- Quality certifications (e.g., GMP, GLP, GDP, ISO 9001, ISO 13485, ISO 17025).
- On-site audit results and third-party inspection records.
- Annual quality reviews, regular document updates, and audits.
We also assess suppliers’ process capabilities, product quality, on-time delivery performance, and collaboration through supplier self-assessment questionnaires, document reviews, and site audits. These efforts are meant to strengthen partnerships and create value.
New Supplier Management
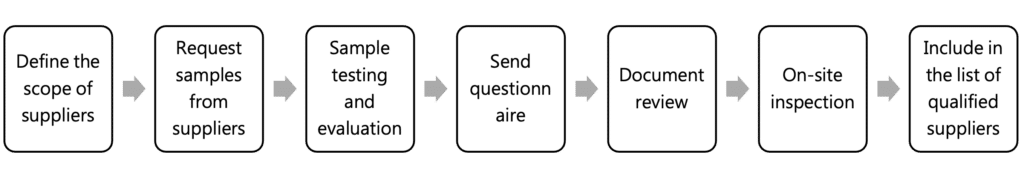
New suppliers follow a 7-step evaluation process: defining scope, testing samples, gathering questionnaires, and conducting reviews and site visits. Approved suppliers are added to our qualified list, ensuring reliable supply and quality standards.
Supply Chain Review Methods
Bora implements a multi-level review process to ensure suppliers maintain stable supply capacity and product quality.
A. Basic Review and Quotation
- Based on procurement value, suppliers must provide quotes from at least two to three vendors. Designated suppliers require justification and supervisor approval.
- For special items such as fixed asset procurement or patented products, project-specific reviews may be conducted.
B. Supplier Basic Information and Document Review
- Verify supplier registration details, payment terms and contract contents to ensure they meet company requirements.
- Request suppliers to provide Certificates of Analysis (COA), technical data, and quality questionnaires to assess product suitability.
C. Sample Testing and Evaluation
- Test and evaluate supplier-provided samples to ensure compliance with t company standards technical capabilities.
D. Formal Evaluation and Audit
- Conduct a comprehensive review of suppliers following internal quality procedures, including document reviews and on-site inspections when necessary.
- Assess supplier performance in quality management, product performance, supply stability, and delivery punctuality.
E. Qualified Supplier Management
- Approved suppliers are added to the qualified supplier list and are subject to regular quality reviews and document reassessments.
- Ensure stable, t long-term collaboration and enhance supply chain resilience.
By applying consistent review standards, Bora ensures a reliable, high-quality, and competitive supply chain.
